Developing Successful FDA Compliant Medical Devices Software and Firmware for Safety-Critical Systems Today, medical devices rely on an increasing amount of software running on embedded microprocessors, PCs, smartphones, and the cloud. Whether you are designing and developing standalone Software as a medical device (SaMD), networked medical devices, a component of an IoT system, or a […]
Arguably one of the largest concerns regarding the Internet of Things (IoT), cybersecurity is a hot topic when it comes to developing a new product. Products connected to the IoT, or communicating with a larger network of devices, are often vulnerable to external technical attacks. These attacks cause the device to malfunction or work against […]
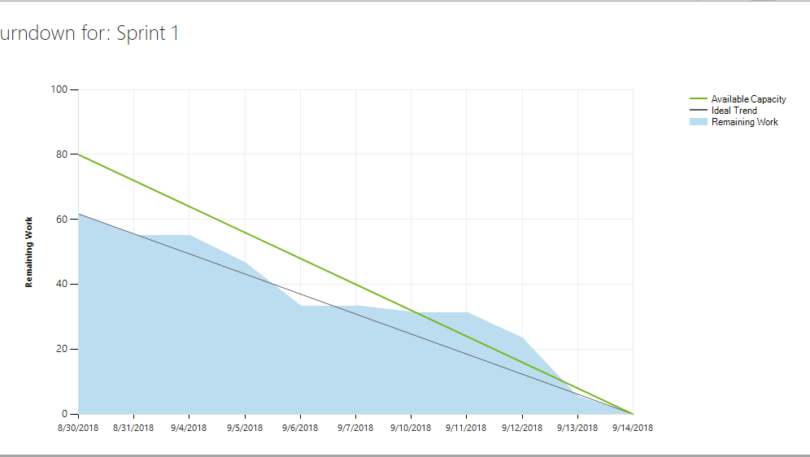
The Master Test Plan (MTP) defines the general approach to testing at the architectural level within a device’s software. This document applies to the product’s software testing as a complete platform. It addresses testing of the product’s software and its components to understand the extent of testing necessary to ensure the software performs as required. […]
The Software Development Plan is the planning document for the development of software and software support for a product. It provides an overview of software development, describes how the project is managed, how work is conducted, and how the development process is tailored to meet applicable standards, requirements, and objectives. This includes a description of […]
During the project planning phase, the two most important components are the product requirements and the architectural design. These components create a clear vision for how the product should be engineered. The goal of the architectural design specification (ADS) document is to define the platform software architecture and to provide an assessment of the impact […]
The purpose of a Quality Assurance Plan is to define the general approach taken to ensure quality throughout the product development process. It should cover all basic information concerning review, development, and planning for verification, validation, and proving the system can provide the level of support required by the product while minimizing the chance of failure. […]
After establishing sound requirements, as described in Part 4, utilize each requirement as part of your work breakdown structure (WBS). A WBS essentially breaks down each requirement, or deliverable, into easier-to-manage components in which enables the project manager to develop a schedule and budget for each piece. With a complete WBS, the project team can […]
The purpose of product / software requirements is to identify the true needs of the product and bring these requirements to life by engineering the software properly, the first time. Requirements are a best practice of quality software engineering and extremely vital to project success. Without an organized and concise format, a non-technical project champion […]
The purpose of a risk management plan is to provide assurance that all project risks are identified and analyzed, and options / solutions are presented to eliminate or reduce the effect of those risks. It allows project stakeholders to evaluate these risks and provides an opportunity to add or modify the risks identified and solutions […]
The purpose of a Master Project Plan (MPP) is to provide assurance that the product in development is engineered to industry standard regulations and to act as a guide for the development team, ultimately resulting in a successful and satisfactory implementation. To begin, consider the key elements needed to complete a project plan. Most MPPs […]